1. Introduction to Pharmaceutical Plastic Packaging Materials
1.1 What Are Pharmaceutical Plastic Packaging Materials
Plastic is a shortened term for thermoplastic polymers, primarily composed of two components: resins and chemical additives. It is one of the primary materials used in pharmaceutical packaging. Compared to glass packaging materials, plastic packaging materials offer advantages such as lightweight properties, high mechanical strength, corrosion resistance, ease of sealing, and lower costs. As a result, they have been widely adopted in pharmaceutical packaging in recent years.
1.2 Characteristics of Pharmaceutical Plastic Packaging Materials
(1) Excellent mechanical properties. They possess good strength and elasticity, capable of withstanding bending, impact, and compression, as well as being resistant to friction and breakage; (2) Good chemical stability. Compared to the hydrolytic properties of glass packaging materials and the corrosion properties of metal packaging materials, plastic packaging materials have better chemical stability, exhibiting good resistance to common acids, alkalis, and salts, as well as excellent resistance to chemical media such as oxygen, moisture, and carbon dioxide in the external packaging environment; (3) Lightweight. Plastic packaging materials are generally lightweight, which is related to their low density. Their density is approximately half that of glass and one-fifth that of metal; (4) Excellent processability. Plastic is heat-sensitive and highly malleable, making it easy to form, heat-seal, and laminate, as well as print and decorate; (5) Good optical properties. Packaging materials can be made transparent or opaque depending on the light sensitivity of the drug; (6) Low cost. Plastic packaging materials are relatively inexpensive and, due to their lightweight nature, have lower transportation costs.
2. Types and Advantages/Disadvantages of Pharmaceutical Plastic Packaging Materials
2.1 Polyethylene (PE)
Polyethylene, abbreviated as PE, can be classified into linear low-density polyethylene (LLDPE), low-density polyethylene (LDPE), medium-density polyethylene (MDPE), and high-density polyethylene (HDPE) based on differences in density and structure. Different densities of polyethylene have distinct properties: High-density polyethylene (HDPE) is a relatively hard and tough material with strong resistance to various chemicals, lower transparency, and good barrier properties; Low-density polyethylene (LDPE) is soft, transparent, and has good heat sealability, but poor barrier properties against gases or odors; Linear low-density polyethylene (LLDPE) has better toughness, elasticity, and barrier properties superior to LDPE. It is thinner, reduced by 20% compared to low-density polyethylene, enabling the production of softer, thinner films with excellent heat sealability.

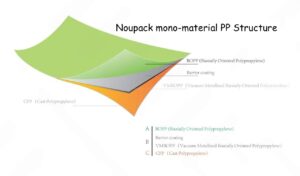
2.2 Polypropylene (PP)
Polypropylene is a high-molecular-weight polymer of propylene, with an appearance similar to polyethylene, but it has a lower density and is lighter than polyethylene, making it the lightest plastic packaging material currently available. Polypropylene materials have high chemical resistance; superior mechanical properties, with mechanical properties superior to PE, especially in terms of bending resistance and rigidity; higher transparency than PE; good moisture resistance and better gas barrier properties than PE, effectively preventing odor transmission; good heat resistance, resistant to boiling water, and suitable for packaging materials requiring high-temperature sterilization; non-toxic and odorless. However, this material also has some drawbacks. Polypropylene is brittle at low temperatures and has significantly poorer cold resistance than PE, making it unsuitable for use in low-temperature environments; it has relatively weak aging resistance, inferior to PE, often requiring the addition of antioxidants; and it has poor printability.
2.3 Polyvinyl Chloride (PVC)
Polyvinyl chloride is produced by the polymerization of polyvinyl chloride monomers. This material is widely used in the packaging of solid dosage forms. Currently, a large proportion of the aluminum-plastic blister packaging materials for tablets and capsules on the market are made of PVC. PVC products have good transparency, high strength, and excellent printability, effectively meeting the moisture-proof, antibacterial, and anti-fogging requirements for tablets, capsules, and traditional Chinese medicine formulations. PVC itself is non-toxic, but polyvinyl chloride monomers have hepatocarcinogenic effects. Therefore, when using PVC sheets for pharmaceutical packaging, the content of polyvinyl chloride monomers should be controlled below one part per million. On the other hand, PVC has poor heat resistance and tends to deform when heated, often requiring the addition of stabilizers and plasticizers to lower processing temperatures and adjust the hardness of PVC. Pharmaceutical-grade PVC sheets should use non-toxic additives.
2.4 Polyvinylidene Chloride (PVDC)
Polyvinylidene chloride is produced by the polymerization of vinylidene chloride (VDC) and polyethylene (VC). PVDC material exhibits excellent printability, good transparency, superior heat sealability, and chemical resistance. Its most notable feature is extremely low water and oxygen permeability, making it an excellent high-barrier material. However, this material also has some drawbacks: its thermal stability is inferior to that of PVC; it has poor aging resistance; and the residual polyethylene monomers are toxic, posing risks of teratogenicity and carcinogenicity with long-term exposure. Therefore, when used as pharmaceutical packaging material, strict control is required, with the polyethylene monomer content not exceeding 0.003%. Compared to PE and PP, PVDC is relatively expensive. In pharmaceutical packaging, it is primarily used in combination with PE and PP to form composite film materials, enhancing the film’s gas barrier and moisture barrier properties to improve packaging performance in terms of moisture resistance, oxygen barrier, and sealing.

2.5 Polyester (PET)
Polyester is a polymer containing ester bonds. The type commonly used in pharmaceutical plastic packaging materials is polyethylene terephthalate (PET). This material has excellent mechanical properties, with the highest toughness among commonly used thermoplastic plastics. It exhibits strong tensile and impact resistance, with film tensile strength comparable to aluminum foil and impact resistance 3–5 times that of general films. It has good fold resistance but poor tear resistance; it also has good low-temperature resistance, heat resistance are both good; it has good chemical resistance but is not resistant to concentrated acids or alkalis; it has good gas barrier properties and is classified as a medium-barrier material; it is non-toxic and odorless with good safety; it has high transparency and good gloss, and provides good UV protection. However, this material has poor heat resistance and degrades easily in hot water; it cannot withstand high-temperature steam sterilization; it generates static electricity; and it has poor heat sealability.
3. Applications and precautions for pharmaceutical plastic packaging materials
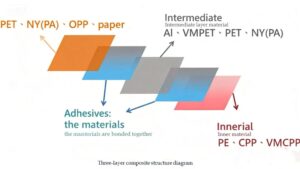
Plastic is easy to process and can be made into plastic bottles and bags of various specifications and shapes. It can also be combined with various packaging materials to form high-performance composite packaging materials. Pharmaceutical plastics are lightweight, high-strength, shatter-resistant, have good sealing properties, are moisture-proof, and hygienic. They can be used directly for pharmaceutical packaging without washing or drying, making them an excellent pharmaceutical packaging container. They are widely used for packaging oral solid dosage forms (such as tablets, granules, capsules, etc.) and liquid dosage forms (such as syrups, etc.). Although plastic has many advantages, it should be selected based on the characteristics of the formulation when used.
For example, when producing plastic bottles for solid drugs, titanium dioxide or white masterbatch is generally added to make the bottles white and opaque. For liquid drugs or those requiring transparency, brown or other colored masterbatch is generally added to meet transparency requirements while also imparting a certain color to the bottle surface to block sunlight. Due to the inherent characteristics of plastic packaging materials, their barrier properties are relatively weak. If the product contains volatile components, they may permeate through the container walls, leading to loss. On the other hand, during production, plastic packaging materials may incorporate various additives, such as plasticizers, UV stabilizers, heat stabilizers, lubricants, antioxidants, colorants, and anti-static agents. When using such materials, it is essential to verify the compatibility of these additives with the medication and assess any potential toxicity or irritation.
4. Summary
Plastic packaging materials are diverse and can be selected and applied based on the characteristics of the drug. For soft packaging, low-density polyethylene can be chosen. For drugs requiring waterproof properties, high-density polyethylene and polypropylene can be selected; for drugs sensitive to oxygen, high-barrier polyvinylidene chloride composite materials can be chosen. For oral liquid formulations, if glass bottles are not selected, polyester packaging is an excellent alternative. Plastic bags for intravenous infusion can rely on their own tension to squeeze out the medication, eliminating the need for an air circuit, thereby avoiding secondary contamination caused by glass bottles. They are an excellent alternative to glass infusion bottles.